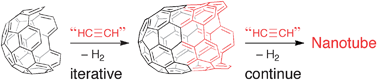
Carbon nanotubes from short hydrocarbon templates. Energy analysis of the Diels–Alder cycloaddition/rearomatization growth strategy†
Abstract
Molecular orbital calculations at the
- This article is part of the themed collection: Celebrating the 70th birthday of Professor Fred Wudl

 Please wait while we load your content...
Please wait while we load your content...