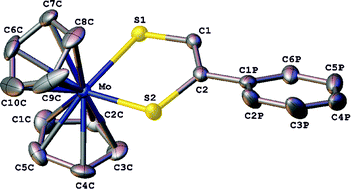
The compounds [Cp2M(S2C2(H)R)] (M = Mo or W; R = phenyl, pyridin-2-yl, pyridin-3-yl, pyridin-4-yl or quinoxalin-2-yl) and [Cp2Mo(S2C2(Me)(pyridin-2-yl)] have been prepared by a facile and general route for the synthesis of dithiolene complexes, viz. the reaction of [Cp2MCl2] (M = Mo or W) with the dithiolene pro-ligand generated by reacting the corresponding 4-(R)-1,3-dithiol-2-one with CsOH. These Mo compounds were reported previously (Hsu et al., Inorg. Chem. 1996, 35, 4743); however, the preparative method employed herein is more versatile and generates the compounds in good yield and all of the W compounds are new. Electrochemical investigations have shown that each compound undergoes a diffusion controlled one-electron oxidation (OXI) and a one-electron reduction (REDI) process; each redox change occurs at a more positive potential for a Mo compound than for its W counterpart. The mono-cations generated by chemical or electrochemical oxidation are stable and the structures of both components of the [Cp2Mo(S2C2(H)R)]+/[Cp2Mo(S2C2(H)R)] (R = Ph or pyridin-3-yl) redox couples have been determined by X-ray crystallography. For each redox related pair, the changes in the Mo–S, S–C and C–C bond lengths of the {MoSCCS} moiety are generally consistent with OXI involving the loss of an electron from a π-orbital that is Mo–S and C–S antibonding and C–C bonding in character. These results have been interpreted successfully within the framework provided by DFT calculations accomplished for [Cp2M(S2C2(H)Ph)]n (M = Mo or W; n = +1, 0 or −1). The HOMO of the neutral compounds is derived mainly from the dithiolene π3 orbital (65%); therefore, OXI is essentially a dithiolene-based process. The similarity of the potentials for OXI (ca. 30 mV) for analogous Mo and W compounds is consistent with this interpretation and the EPR spectra of each of the Mo cations show that the unpaired electron is coupled to the dithiolene proton but relatively weakly to 95,97Mo. The DFT calculations indicate that the unpaired electron is more localised on the metal in the mono-anions than in the mono-cations. In agreement with this, the EPR spectrum of each of the Mo-containing mono-anions manifests a larger 95,97Mo coupling (Aiso) than observed for the corresponding mono-cation and REDI for a W compound is significantly (ca. 300 mV) more negative than that of its Mo counterpart. [Cp2W(S2C2(H)(quinoxalin-2-yl))] is anomalous; REDI occurs at a potential ca. 230 mV more positive than expected from that of its Mo counterpart and the EPR spectrum of the mono-anion is typical of an organic radical. DFT calculations indicate that these properties arise because the electron is added to a quinoxalin-2-yl π-orbital.

 Please wait while we load your content...
Please wait while we load your content...