From insertion of rhodium acetate paddlewheels into functionalized 7-azaindole hydrogen-bonded dimers to infinite architectures†
Abstract
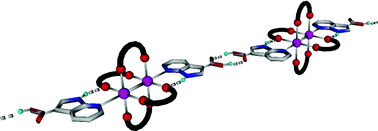
In order to take advantage of the peculiar mode of interaction consisting in the combination of hydrogen and coordination bonding displayed by the 7-azaindole core towards tetraacetate paddlewheel type complexes, a series of new derivatives, bearing peripheral interacting sites at the position 3, have been prepared and their

 Please wait while we load your content...
Please wait while we load your content...