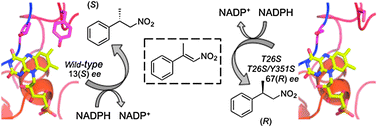
Active site modifications in pentaerythritol tetranitrate reductase can lead to improved product enantiopurity, decreased by-product formation and altered stereochemical outcome in reactions with α,β-unsaturated nitroolefins
Abstract
This work describes a site-directed mutagenesis study of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vs.
C vs.

 Please wait while we load your content...
Please wait while we load your content...