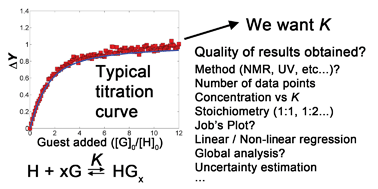
The most common approach for quantifying interactions in supramolecular chemistry is a titration of the guest to solution of the host, noting the changes in some physical property through NMR, UV-Vis, fluorescence or other techniques. Despite the apparent simplicity of this approach, there are several issues that need to be carefully addressed to ensure that the final results are reliable. This includes the use of non-linear rather than linear regression methods, careful choice of stoichiometric binding model, the choice of method (e.g., NMRvs.UV-Vis) and concentration of host, the application of advanced data analysis methods such as global analysis and finally the estimation of uncertainties and confidence intervals for the results obtained. This tutorial review will give a systematic overview of all these issues—highlighting some of the key messages herein with simulated data analysis examples.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...