On the aromatic stabilization of corannulene and coronene†
Abstract
The application of set of homodesmotic reactions allowed us to estimate the aromatic stabilization energy (
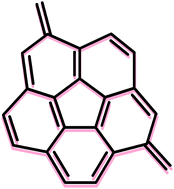
- This article is part of the themed collection: Aromaticity, electron delocalisation, and related molecular properties

 Please wait while we load your content...
Please wait while we load your content...