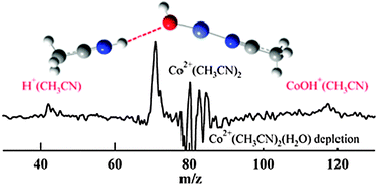
The microsolvation of cobalt and nickel dications by acetonitrile and water is studied by measuring photofragment spectra at 355, 532 and 560–660 nm. Ions are produced by electrospray, thermalized in an ion trap and mass selected by time of flight. The photodissociation yield, products and their branching ratios depend on the metal, cluster size and composition. Proton transfer is only observed in water-containing clusters and is enhanced with increasing water content. Also, nickel-containing clusters are more likely to undergo charge reduction than those with cobalt. The homogeneous clusters with acetonitrile M2+(CH3CN)n (n = 3 and 4) dissociate by simple solvent loss; n = 2 clusters dissociate by electron transfer. Mixed acetonitrile/water clusters display more interesting dissociation dynamics. Again, larger clusters (n = 3 and 4) show simple solvent loss. Water loss is substantially favored over acetonitrile loss, which is understandable because acetonitrile is a stronger ligand due to its higher dipole moment and polarizability. Proton transfer, forming H+(CH3CN), is observed as a minor channel for M2+(CH3CN)2(H2O)2 and M2+(CH3CN)2(H2O) but is not seen in M2+(CH3CN)3(H2O). Studies of deuterated clusters confirm that water acts as the proton donor. We previously observed proton loss as the major channel for photolysis of M2+(H2O)4. Measurements of the photodissociation yield reveal that four-coordinate Co2+ clusters dissociate more readily than Ni2+ clusters whereas for the three-coordinate clusters, dissociation is more efficient for Ni2+ clusters. For the two-coordinate clusters, dissociation is viaelectron transfer and the yield is low for both metals. Calculations of reaction energetics, dissociation barriers, and the positions of excited electronic states complement the experimental work. Proton transfer in photolysis of Co2+(CH3CN)2(H2O) is calculated to occur via a (CH3CN)Co2+–OH−–H+(NCCH3) salt-bridge transition state, reducing kinetic energy release in the dissociation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...