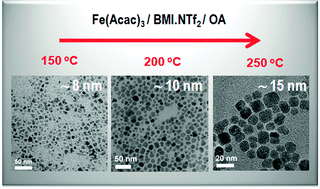
This work describes an easy synthesis (one pot) of MFe2O4 (M = Co, Fe, Mn, and Ni) magnetic nanoparticles MNPs by the thermal decomposition of Fe(Acac)3/M(Acac)2 by using BMI·NTf2 (1-n-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide) or BMI·PF6 (1-n-butyl-3-methylimidazolium hexafluorophosphate) ionic liquids (ILs) as recycling solvents and oleylamine as the reducing and surface modifier agent. The effects of reaction temperature and reaction time on the features of the magnetic nanomaterials (size and magnetic properties) were investigated. The growth of the MNPs is easily controlled in the IL by adjusting the reaction temperature and time, as inferred from Fe3O4 MNPs obtained at 150 °C, 200 °C and 250 °C with mean diameters of 8, 10 and 15 nm, respectively. However, the thermal decomposition of Fe(Acac)3 performed in a conventional high boiling point solvent (diphenyl ether, bp 259 °C), under a similar Fe to oleylamine molar ratio used in the IL synthesis, does not follow the same growth mechanism and rendered only smaller NPs of 5 nm mean diameter. All MNPs are covered by at least one monolayer of oleylamine making them readily dispersible in non-polar solvents. Besides the influence on the nanoparticles growth, which is important for the preparation of highly crystalline MNPs, the IL was easily recycled and has been used in at least 20 successive syntheses.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...