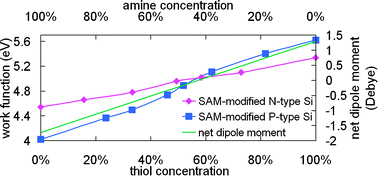
It has been shown that the application of self-assembled monolayers (SAMs) to semiconductors or metals may enhance the efficiency of optoelectronic devices by changing the surface properties and tuning the work functions at their interfaces. In this work, binary SAMs with various ratios of 3-aminopropyltrimethoxysilane (APTMS) and 3-mercaptopropyltrimethoxysilane (MPTMS) were used to modify the surface of Si to fine-tune the work function of Si to an arbitrary energy level. As an electron-donor, amine SAM (from APTMS) produced outward dipole moments, which led to a lower work function. Conversely, electron-accepting thiol SAM (from MPTMS) increased the work function. It was found that the work function of Si changed linearly with the chemical composition and increased with the concentration of thiol SAMs. Because dipoles of opposite directions cancelled each other out, homogeneously mixing them leads to a net dipole moment (hence the additional surface potential) between the extremes defined by each dipole and changes linearly with the chemical composition. As a result, the work function changed linearly with the chemical composition. Furthermore, the amine SAM possessed a stronger dipole than the thiol SAM. Therefore, the SAMs modified with APTMS showed a greater work function shift than did the SAMs modified with MPTMS.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...