Deuterium kinetic isotope effects on the gas-phase reactions of C2H with H2(D2) and CH4(CD4)
Abstract
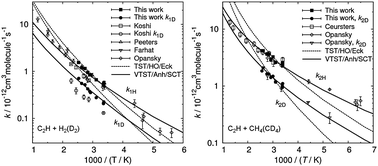
Kinetics of the ethynyl (C2H) radical reactions with H2, D2, CH4 and CD4 was studied over the temperature range of 295–396 K by a pulsed laser photolysis/chemiluminescence technique. The C2H radicals were generated by ArF excimer-laser photolysis of C2H2 or CF3C2H and were monitored by the chemiluminescence of CH(A2Δ) produced by their reaction with O2 or O(3P). The measured absolute rate constants for H2 and CH4 agreed well with the available literature data. The primary kinetic isotope effects (KIEs) were determined to be kH2/kD2 = 2.48 ± 0.14 and kCH4/kCD4 = 2.45 ± 0.16 at room temperature. Both of the KIEs increased as the temperature was lowered. The KIEs were analyzed by using the variational transition state theory with semiclassical small-curvature tunneling corrections. With anharmonic corrections on the loose transitional vibrational modes of the transition states, the theoretical predictions satisfactorily reproduced the experimental KIEs for both C2H + H2(D2) and C2H + CH4(CD4) reactions.

 Please wait while we load your content...
Please wait while we load your content...