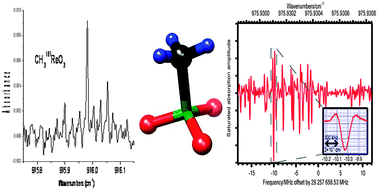
Originating from the weak interaction, parity violation in chiral molecules has been considered as a possible origin of biohomochirality. We have proposed the observation of molecular parity violation using the two-photon Ramsey fringes technique on a supersonic beam. As a first step in this direction, a detailed spectroscopic study of methyltrioxorhenium (MTO) has been undertaken. It is an ideal test molecule as the achiral parent molecule of chiral candidates for a parity violation experiment. For the 187Re MTO isotopologue, a combined analysis of Fourier transform microwave and infrared spectra as well as ultra-high resolution CO2 laser absorption spectra enabled the assignment of 28 rotational lines and 71 rovibrational lines, some of them with a resolved hyperfine structure. A set of spectroscopic parameters in the ground and first excited state, including hyperfine structure constants, was obtained for the νas antisymmetric Re![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode of this molecule. This result validates the experimental approach to be followed once a chiral derivative of MTO is synthesized, and shows the benefit of the combination of several spectroscopic techniques in different spectral regions, with different set-ups and resolutions. The first high resolution spectra of jet-cooled MTO, obtained on a set-up being developed for the observation of molecular parity violation, are shown, which constitutes a major step towards the targeted objective.
O stretching mode of this molecule. This result validates the experimental approach to be followed once a chiral derivative of MTO is synthesized, and shows the benefit of the combination of several spectroscopic techniques in different spectral regions, with different set-ups and resolutions. The first high resolution spectra of jet-cooled MTO, obtained on a set-up being developed for the observation of molecular parity violation, are shown, which constitutes a major step towards the targeted objective.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode of this molecule. This result validates the experimental approach to be followed once a chiral derivative of MTO is synthesized, and shows the benefit of the combination of several spectroscopic techniques in different spectral regions, with different set-ups and resolutions. The first high resolution
O stretching mode of this molecule. This result validates the experimental approach to be followed once a chiral derivative of MTO is synthesized, and shows the benefit of the combination of several spectroscopic techniques in different spectral regions, with different set-ups and resolutions. The first high resolution 

 Please wait while we load your content...
Please wait while we load your content...