The aqueous phase behavior of polyion–surfactant ion complex salts mixed with nonionic surfactants†‡
Abstract
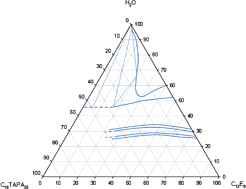
The aim of this work was to study intermolecular interactions in systems containing charged polyion (polyacrylate, PA−), charged
- This article is part of the themed collection: scattering methods applied to soft matter

 Please wait while we load your content...
Please wait while we load your content...