Dimensional caging of polyiodides: cation-templated synthesis using bipyridinium salts†
Abstract
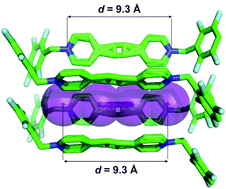
The potential of bipyridinium derivatives in the cation templated synthesis of polyiodides has been explored by applying the strategy of size-matching between
- This article is part of the themed collection: Dynamic behaviour and reactivity in crystalline solids

 Please wait while we load your content...
Please wait while we load your content...