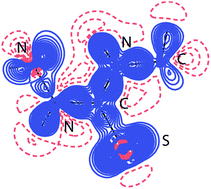
Sulfur atom is generally considered as a weak hydrogen bonding acceptor which is mostly explained by its low electronegativity. This is a background, and motivation, for this study that aims to provide additional insight into electronic features of the sulfur acceptor. The analysis of sulfur's electronic features uses high-resolution, low-temperature X-ray diffraction datasets on 4-methyl-3-thiosemicarbazide (MeTSC) and salicylaldehyde thiosemicarbazone (SalTSC). The total electron density distribution was modeled using Hansen–Coppens multipole model. The fact that MeTSC crystallizes with two crystallographically independent (but chemically equivalent) molecules, engaging in partly different D–H⋯S interactions, has, quite conveniently, enabled the examination of fine effects of the D–H⋯S interactions on the lone pairs electron density of the two S acceptors. The deformation density distribution, topological analysis of the total experimental charge density ρ(r) and its Laplacian (∇2ρ(r)), and, finally, electrostatic potential all point to the great flexibility of the S acceptor and its ability to adjust to the spatial distribution of the interacting donor groups. Toroidal accumulation of the S lone pairs electron density, strong value of the negative electrostatic potential even at a distance of 3.1 Å from the S nucleus, and interpenetration of the van der Waals spheres for the donor and S acceptor atoms are all an indication of sulfur's capacity to simultaneously engage in a greater number of interactions than conventional acceptors such as O and N.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...