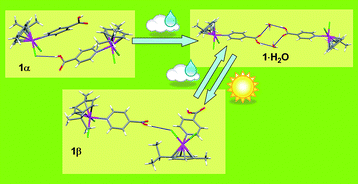
The X-ray structure of the half-sandwich Ru(II) complex [(p-cymene)Ru(κN-INA)Cl2] (INA = isonicotinic acid) (1α) does not show the expected cyclic dimerization of the carboxylic functions of INA. The good hydrogen bond accepting character of the chloride ligand in fact, leads to the formation of Ru–Cl⋯HOOC intermolecular hydrogen bonded chains. Interestingly, 1α quickly converts into the hydrated form [(p-cymene)Ru(κN-INA)Cl2]H2O (1·H2O) once exposed to water vapours through a heterogeneous solid-gas reaction. In the X-ray structure of 1·H2O two water molecules bridge two carboxylic functions of two different organometallic entities, giving rise to a R44(12) cyclic dimer. The 1α to 1·H2O transformation occurs smoothly even when the first is exposed to vapours of absolute ethanol, whose water content is not higher than 0.2%. The thermally induced dehydration of 1·H2O does not give 1α back, but rather it leads to a not yet defined transient species (1γ) which finally transforms into a polymorphic form of 1α, whose solid state structure has been solved by X-ray powder diffraction analysis (1β).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...