The supramolecular structures of eight aryl protected ethyl-6-methyl-4-phenyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates have been analyzed to determine the role of different functional groups on the molecular geometry, conformational characteristics and the packing of these molecules in the crystal lattice. Out of these the parafluoro substituted compound on the aryl ring exhibits conformational polymorphism, due to the different conformation of the ester moiety. This behaviour has been characterized using both powder and single-crystal X-ray diffraction, optical microscopy and differential scanning calorimetry performed on both these polymorphs. The compounds pack via the cooperative interplay of strong N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C intermolecular dimers and chains forming a sheet like structure. In addition, weak C–H⋯O
C intermolecular dimers and chains forming a sheet like structure. In addition, weak C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H⋯π interactions impart additional stability to the crystal packing.
C and C–H⋯π interactions impart additional stability to the crystal packing.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C intermolecular dimers and chains forming a sheet like structure. In addition, weak C–H⋯O
C intermolecular dimers and chains forming a sheet like structure. In addition, weak C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H⋯π interactions impart additional stability to the crystal packing.
C and C–H⋯π interactions impart additional stability to the crystal packing.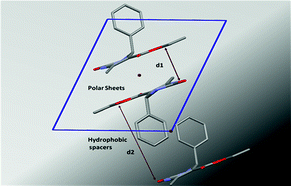

 Please wait while we load your content...
Please wait while we load your content...