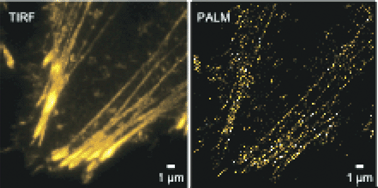
Photoswitchable fluorophores play an essential role in super-resolution fluorescence microscopy, including techniques such as photoactivated localization microscopy (PALM). A determining factor in the precision of the images generated by PALM measurements is the photon numbers that can be detected from the fluorophores. Dronpa is a reversibly photoswitchable fluorescent protein that has been successfully used in PALM experiments. The number of photons per switching cycle that can be acquired for Dronpa depends on its off-switching rate, limiting the number of photons that can be recorded. In this study we report our discovery that the tetrameric ancestor of Dronpa, 22G, shows slower switching, and develop a mutant that displays switching kinetics between those of Dronpa and 22G. We show that the kinetics of the photoswitching are strongly related to self-association of the protein, supporting our view of dynamic flexibility as determining in the photoswitching. Similarly we find that higher-resolution PALM images can be acquired with slower-switching proteins due to their higher number of emitted photons per switching cycle.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...