Damien Habrant and Ari M. P. Koskinen
Org. Biomol. Chem., 2010,8, 4364-4373
DOI:
10.1039/C0OB00092B,
Paper
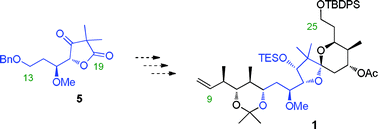
An asymmetric synthesis of the C9–C25 spiroketal fragment of