Chiral phosphine oxides in present-day organocatalysis†
Abstract
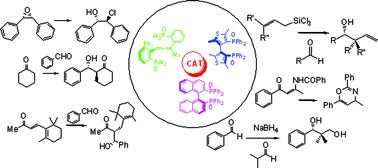
The design and synthesis of new chiral Lewis bases is a field of extraordinary activity; in this context, while N-oxides derived from both N-heterocyclic systems and aliphatic amines have found widespread applications in organocatalysis, quite surprisingly phosphine oxides have been used less frequently. This contribution will highlight the relatively few examples of stereoselective transformations organocatalyzed by chiral phosphine oxides, discussing the different proposed reaction mechanisms and identifying topics for future investigation in what can be most certainly defined as an “Emerging Area”.

 Please wait while we load your content...
Please wait while we load your content...