Reverse-direction (5′→3′) synthesis of oligonucleotides containing a 3′-S-phosphorothiolate linkage and 3′-terminal 3′-thionucleosides†
Abstract
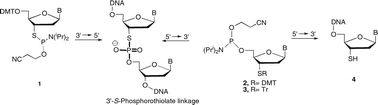
The synthesis of oligodeoxynucleotides containing 3′-thionucleosides has been explored using a reverse-direction (5′→3′) approach, based on nucleoside monomers which contain a trityl- or dimethoxytrityl-protected 3′-thiol and a 5′-O-phosphoramidite. These monomers are relatively simple to prepare as trityl-based protecting groups were introduced selectively at a 3′-thiol in preference to a 5′-hydroxyl group. As an alternative approach, trityl group migration could be induced from the 5′-oxygen to the 3′-thiol function. 5′→3′ Synthesis of oligonucleotides gave relatively poor yields for the internal incorporation of 3′-thionucleosides [to give a 3′-S-phosphorothiolate (3′-SP) linkage] and multiple 3′-SP modifications could not be introduced by this method. However, the reverse direction approach provided an efficient route to oligonucleotides terminating with a 3′-thionucleoside. The direct synthesis of these thio-terminating oligomers has not previously been reported and the methods described are applicable to 2′-deoxy-3′-thionucleosides derived from thymine, cytosine and adenine.

 Please wait while we load your content...
Please wait while we load your content...