Metallic cobalt/cobalt sulfide hetero-nanostructures embedded within N-doped graphitic carbon nanocages for the hydrogen evolution reaction†
Abstract
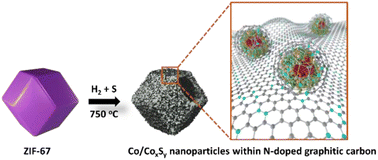
The development of low-cost, catalytically efficient, and durable earth-abundant electrocatalysts for replacing platinum-group-metal (PGM) materials has always been at the forefront of materials engineering for sustainable hydrogen generation. Metal–organic frameworks (MOFs), especially cobalt (Co)-based zeolitic imidazolate framework (ZIF-67) can be used as precursors or templates for preparing non-precious electrocatalysts. Herein, we successfully fabricated metallic cobalt/cobalt sulfide hetero-nanostructures embedded within N-doped graphitic carbon nanocages (Co/CoxSy@NC-750) by the one-step pyrolysis of ZIF-67 under H2 and S. Co/CoxSy@NC-750 exhibits a hollow configuration with a high degree of morphological uniformity. The pyrolysis temperature and resulting control of the composition played a crucial role in material engineering with application-oriented properties. Examination of the activity for the hydrogen evolution reaction (HER) in 0.5 M H2SO4 and 1.0 M KOH aqueous electrolytes demonstrated that Co/CoxSy@NC-750 exhibits substantially improved HER performance in both acidic (overpotential of 130 mV and Tafel slope of 82 mV dec−1) and basic (330 mV and 160 mV dec−1) media. The unique metallic cobalt core/cobalt sulfide shell is particularly beneficial for maintaining the electrochemical long-term durability (≥30 h and 40 h in acids and bases, respectively). This study provides guidance for achieving MOF-derived inorganic nanomaterials with the desired structural heterogeneity and compositional control for non-PGM electrochemical catalysis through fundamental quantification of the structural and catalytic parameters.


 Please wait while we load your content...
Please wait while we load your content...