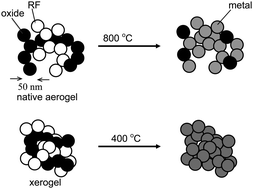
This study establishes that the necessary and sufficient condition for efficient reaction between nanoparticles includes both high surface-to-volume ratios and high compactness. For this, a wide range of interpenetrating networks of resorcinol-formaldehyde (RF) and metal oxide (MOx, M: Fe, Co, Ni, Sn, Cu, Cr, Ti, Hf, Y, Dy) nanoparticles were synthesized via a simple one-pot process using the acidity of gelling solutions of hydrated metal ions to catalyze gelation of RF. The compactness of the nanoparticles in the dry composites is controlled by the drying method: supercritical fluid (SCF) CO2 drying affords aerogels with open skeletal frameworks, while drying under ambient pressure yields much more compact xerogels. A second independent method to impart compactness is by crosslinking the framework nanoparticles with a conformal polyurea (PUA) coating followed by drying with SCF CO2: although those materials (X-aerogels) have an open aerogel-like structure, upon heating in the 200 °C range, the conformal PUA coating melts and causes local structural collapse of the underlying framework creating macropores defined by xerogel-like walls. Depending on the chemical identity of the metal ion, pyrolysis at higher temperatures sets off carbothermal processes yielding pure metal monolithic nanostructures (up to 800 °C; cases of M; Fe, Co, Ni, Sn, Cu) or carbides (up to 1400 °C; cases of M: Cr, Ti, Hf). Irrespective of the specific chemical processes responsible for those transformations, the rate determining factor is the innate compactness of the xerogels, or the induced skeletal compactness in X-aerogels: both kind of materials react at as much as 400 °C lower temperatures than their corresponding native aerogels. By comparison, bulk (micron size) mixtures of the corresponding oxides and carbon black remained practically unreacted in the entire temperature range used for the nanoparticle networks. In addition to the significance of the RF-MOx interpenetrating networks in the design of new materials (mesoporous and macroporous monolithic metals and carbides), the effect of compactness on the activation of the carbothermal processes has important implications for process-design engineering.

 Please wait while we load your content...
Please wait while we load your content...