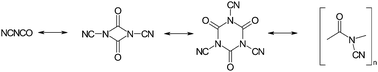
The first inorganic polyisocyanate, (NC–NCO)x, has been prepared and investigated. By polyaddition (polymerisation) of the reactive monomer cyanogen isocyanate (NC–NCO) at low temperatures, the moisture-sensitive polymer was obtained as a phase pure solid. Using several analytical techniques (UV, IR, NMR, MS, DSC, TGA), the formation and the principal connectivity patterns of the hydrogen-free amorphous inorganic network have been settled. From the experimental results, a close structural relation to the organic “nylons” was deduced. Upon thermal impact, this inorganic macromolecule behaves like a typical thermosetting material. Exposing polymeric C2N2O to a temperature between 400 and 500 °C leads to quantitative removal of oxygen in the form of CO2. Depolymerisation and fragmentation at higher temperatures resulted in a virtually complete mass loss due to release of gaseous species. Some preliminary results concerning the high-pressure transformation towards a crystalline “C2N2O” (carbon oxynitride) are also given.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...