Solubility in CO2 and carbonation studies of epoxidized fatty aciddiesters: towards novel precursors for polyurethane synthesis†
Abstract
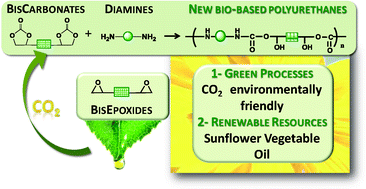
Novel linear polyurethanes were synthesized by bulk
* Corresponding authors
a
Université de Bordeaux, Laboratoire de Chimie des Polymères Organiques, UMR 5629, IPB/ENSCBP, 16 avenue Pey-Berland, Pessac cedex, France
E-mail:
cramail@enscbp.fr
Fax: (+33)5 4000 8487
Tel: (+33)5 4000 6254
b Centre National de la Recherche Scientifique, Laboratoire de Chimie des Polymères Organiques, Pessac cedex, France
c Université de Bordeaux, Institut des Sciences Moléculaires, UMR 5255, 351, Cours de la Libération, Talence Cedex, France
d ITERG, 11 rue Gaspard Monge, Parc Industriel, Pessac Cedex, France
Novel linear polyurethanes were synthesized by bulk

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
A. Boyer, E. Cloutet, T. Tassaing, B. Gadenne, C. Alfos and H. Cramail, Green Chem., 2010, 12, 2205 DOI: 10.1039/C0GC00371A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
