Towards a greener synthesis of (S)-3-aminobutanoic acid: process development and environmental assessment†
Abstract
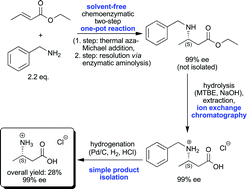
An improved, greener process for the enantioselective chemoenzymatic synthesis of (S)-3-aminobutanoic acid has been developed. Reaction steps comprise an initial aza-Michael addition starting from cheap prochiral compounds, subsequent enzymatic resolution via aminolysis using commercially available Candida antarctica lipase B in a solvent-free one-pot process, hydrolysis of the resulting ester and removal of the N-benzyl moiety via hydrogenation. After isolation, the desired (S)-3-aminobutanoic acid was obtained in an overall yield of 28% and with an excellent enantiomeric excess of 99% ee. Notably, this reaction sequence does not require column chromatography with organic solvents and only one purification step of an intermediate is needed. The environmental impact of this optimized process has been evaluated and an E-factor of 41 has been calculated for the overall process. A comparative assessment with the previous process was done via mass balancing using the E-factor, the selectivity index S−1 as well as an SHE assessment.

 Please wait while we load your content...
Please wait while we load your content...