Microwave-assisted multicomponent domino cyclization–aromatization: an efficient approach for the synthesis of substituted quinolines†
Abstract
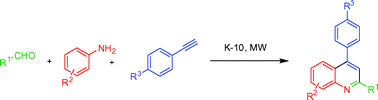
A solid acid-catalyzed microwave-assisted synthesis of substituted quinolines is described. The quinolines were synthesized by a multicomponent domino reaction of anilines, aldehydes and terminal aryl alkynes. The synthetic pathway involves the formation of an

 Please wait while we load your content...
Please wait while we load your content...