Complexes [Ce(NR2)3] (1) or [Ce(NR″2)3] (2) were cerium(III) precursors to the X-ray characterised crystalline oligomeric oxygen-containing amidocerium(IV) compounds [{Ce(NR2)2(μ-O)}n] (3, n = 2; 4, n = 3), [{Ce(NR″2)2(μ-O)}4] (5), [{(R2N)3Ce}2(μ-![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) OMOM
OMOM![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )] (6, M = Na; 7, M = K), [{(R2N)3CeOCe(NR2)2}2(μ-
)] (6, M = Na; 7, M = K), [{(R2N)3CeOCe(NR2)2}2(μ-![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) OKOK
OKOK![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )] (8), and [{Ce(NR2)3}2(μ-η2:η2-O2)]·2CnH2n+2 (9, n = 6; 9′, n = 5) [R = SiMe3, NR″2 = TMP =
)] (8), and [{Ce(NR2)3}2(μ-η2:η2-O2)]·2CnH2n+2 (9, n = 6; 9′, n = 5) [R = SiMe3, NR″2 = TMP = ![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) NC(Me)2(CH2)3C
NC(Me)2(CH2)3C![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) Me2]. Each was isolated in low, or for 5 very low, yield. Except for 4, the oxidising agent was O2 at −27 °C in hexane (3, 6, 7, 8, 9), pentane (9′), or toluene (5), and a co-reagent for the alkali metal bis(trimethylsilyl)amido(oxy)cerate(IV)s was NaNR2 (8) or KNR2 (7, 8). From 1 and an equivalent portion of 2,6-tBu2-benzoquinone after 5 weeks in pentane there was obtained the bis(amido)cyclotricer(IV)oxane 4. The NMR spectral solution chemical shifts for NR2 groups of 3, 4, and 6–9 were consistent with each sample being diamagnetic and hence a Ce(IV) species. A transient amidocerium(IV) superoxide Ce(NR2)3(η2-O2) (J), or its TMP analogue, is considered to be the common first-formed intermediate in each case, while 4 is believed to have arisen from the adventitious hydrolysis of [{Ce(NR2)3O}2(tBu2C6H2-1,4)].
Me2]. Each was isolated in low, or for 5 very low, yield. Except for 4, the oxidising agent was O2 at −27 °C in hexane (3, 6, 7, 8, 9), pentane (9′), or toluene (5), and a co-reagent for the alkali metal bis(trimethylsilyl)amido(oxy)cerate(IV)s was NaNR2 (8) or KNR2 (7, 8). From 1 and an equivalent portion of 2,6-tBu2-benzoquinone after 5 weeks in pentane there was obtained the bis(amido)cyclotricer(IV)oxane 4. The NMR spectral solution chemical shifts for NR2 groups of 3, 4, and 6–9 were consistent with each sample being diamagnetic and hence a Ce(IV) species. A transient amidocerium(IV) superoxide Ce(NR2)3(η2-O2) (J), or its TMP analogue, is considered to be the common first-formed intermediate in each case, while 4 is believed to have arisen from the adventitious hydrolysis of [{Ce(NR2)3O}2(tBu2C6H2-1,4)].
![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) OMOM
OMOM![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )] (6, M =
)] (6, M = ![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) OKOK
OKOK![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) )] (8), and [{Ce(NR2)3}2(μ-η2:η2-O2)]·2CnH2n+2 (9, n = 6; 9′, n = 5) [R =
)] (8), and [{Ce(NR2)3}2(μ-η2:η2-O2)]·2CnH2n+2 (9, n = 6; 9′, n = 5) [R = ![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) NC(Me)2(CH2)3C
NC(Me)2(CH2)3C![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) Me2]. Each was isolated in low, or for 5 very low, yield. Except for 4, the oxidising agent was O2 at −27 °C in
Me2]. Each was isolated in low, or for 5 very low, yield. Except for 4, the oxidising agent was O2 at −27 °C in 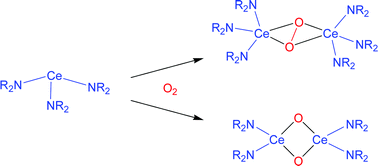

 Please wait while we load your content...
Please wait while we load your content...