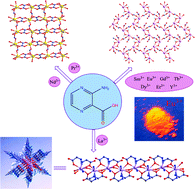
The first example of rare-earth organic frameworks with 3-aminopyrazine-2-carboxylic acid (Hapca) was synthesized under hydrothermal conditions and characterized by elemental analysis, IR, PL, TG, powder and single-crystal X-ray diffraction. These ten complexes exhibit three different structure types with decreasing lanthanide radii: [La(apca)3]n (1) for type I, {[Ln(apca)(ox)(H2O)2]·H2O}n (Ln = Pr (2), Nd (3), ox = oxalate) for type II, and [Ln2(apca)4(OH)2(H2O)2]n (Ln = Sm (4), Eu (5), Gd (6), Tb (7), Dy (8), Er (9), Y (10)) for type III. The structure of type I consists of 1D “snowflake” chains along a-axis, which are further interconnected by hydrogen bonds to produce a 3D sra net topology containing infinite (–C–O–La–)n rod-shaped SBU. Type II has 2D Ln-apca-ox 44-net, in which a planar udud water tetramers (H2O)4 are formed by coordinated and free water molecules. Type III also comprises of 2D 44-layer network constructed from Ln-apca-OH. The structure diversity is mainly caused by the variation of coordinated ligand and lanthanide contraction effect. Remarkably, the oxalate in type II was in situ synthesized from 3-aminopyrazine-2-carboxylic acid through an oxidation-hydrolysis reaction. The luminescent investigations reveal that complex 1 exhibits strong blue emission and complex 5 exhibits characteristic luminescence of Eu3+.