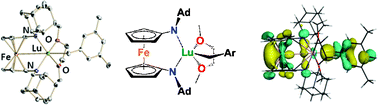
A comparison between the reactivity behavior of two lutetium benzyl complexes supported by different ferrocene-diamide ligands towards aromatic N-heterocycles, such as 1-methylimidazole, isoquinoline, and pyridines, is presented. The two ferrocene-diamide ancillary ligands differ in their nitrogen-donor substituent: adamantyl for one and t-butyldimethylsilyl for the other. The synthesis and characterization of the adamantyl-derived complex 1Ad-DME are reported. The ring opening of 1-methylimidazole by the THF analogue of 1Ad-DME, 1Ad-THF, was observed, analogously to the ring opening of the same substrate by the lutetium benzyl complex supported by the silyl-substituted ligand. Also, analogous products were observed in the reactions with isoquinoline.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...