Low temperature kinetics: the association of OH radicals with O2
Abstract
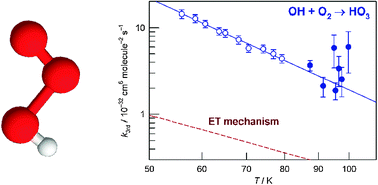
We report the first measurements of rate constants for the reaction in which OH radicals associate with O2 to form HO3. Our recent measurements (Science, 2010, 328, 1258) have shown that the HO–O2 bond dissociation energy is only (12.3 ± 0.3) kJ mol−1. Consequently, above ca. 90 K under attainable experimental conditions, the rate of the reverse dissociation of HO3 becomes comparable to, and then greater than, the rate of the forward association reaction. We have used the CRESU (Cinétique de Réaction en Ecoulement Supersonique Uniforme) method to access low temperatures and have explored the kinetics of OH + O2 + M → HO3 + M in two series of experiments. At temperatures between 55.9 and 79.2 K, the OH radicals, created by pulsed laser photolysis of H2O2 and observed by laser-induced fluorescence, decayed by pseudo-first-order kinetics to effectively zero concentration at longer times. The third-order rate constants derived from these experiments fit the expression: ko3rd(T) = (4.2 ± 1.9) × 10−34 (T/298 K)−(3.5±0.3) cm6 molecule−2 s−1. At temperatures between 87.4 and 99.8 K, rate constants for the association reaction were determined allowing for the significant occurrence of the reverse dissociation reaction. The values of the derived rate constants are consistent with those obtained in the lower temperature range, though the errors are larger. The experimental values of ko3rd(T) are compared with (a) those for other association reactions involving species of similar complexity, and (b) values of ko3rd(T) estimated according to both the energy transfer (ET) and the radical–complex (RC) mechanisms. We conclude that the RC mechanism probably makes the major contribution to the association of OH + O2 at the low temperatures of our experiments.

 Please wait while we load your content...
Please wait while we load your content...