Prediction of the structures of free and oxide-supported nanoparticles by means of atomistic approaches: the benchmark case of nickelclusters
Abstract
The structures of
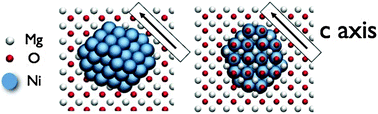
- This article is part of the themed collection: Solid State Structure Prediction

 Please wait while we load your content...
Please wait while we load your content...