This article discusses and compares various methods for defining and measuring radical stability, including the familiar radical stabilization energy (RSE), along with some lesser-known alternatives based on corrected carbon–carbon bond energies, and more direct measures of the extent of radical delocalisation. As part of this work, a large set of R–H, R–CH3, R–Cl and R–R BDEs (R˙ = ˙CH2X, ˙CH(CH3)X, ˙C(CH3)2X and X = H, BH2, CH3, NH2, OH, F, SiH3, PH2, SH, Cl, Br, N(CH3)2, NHCH3, NHCHO, NHCOCH3, NO2, OCF3, OCH2CH3, OCH3, OCHO, OCOCH3, Si(CH3)3, P(CH3)2, SC(CH3)2CN, SCH2COOCH3, SCH2COOCH3, SCH2Ph, SCH3, SO2CH3, S(O)CH3, Ph, C6H4–pCN, C6H4–pNO2, C6H4–pOCH3, C6H4–pOH, CF2CF3, CF2H, CF3, CCl2H, CCl3, CH2Cl, CH2F, CH2OH, CH2Ph, cyclo-CH(CH2)2, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, CH2CH3, CH(CH3)2, C(CH3)3, C
CH2, CH2CH3, CH(CH3)2, C(CH3)3, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, CH
CH, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, CH
CH2, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3, CHO, CN, COCH3, CON(CH2CH3)2, CONH2, CONHCH3, COOC(CH3)3, COOCH2CH3, COOCH3, COOH, COPh), and associated radical stability values are calculated using the high-level ab initio molecular orbital theory method G3(MP2)-RAD. These are used to compare the alternative radical stability schemes and illustrate principal structure–reactivity trends.
CHCH3, CHO, CN, COCH3, CON(CH2CH3)2, CONH2, CONHCH3, COOC(CH3)3, COOCH2CH3, COOCH3, COOH, COPh), and associated radical stability values are calculated using the high-level ab initio molecular orbital theory method G3(MP2)-RAD. These are used to compare the alternative radical stability schemes and illustrate principal structure–reactivity trends.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, CH2CH3, CH(CH3)2, C(CH3)3,
CH2, CH2CH3, CH(CH3)2, C(CH3)3, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH
CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, CH
CH2, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH3,
CHCH3, 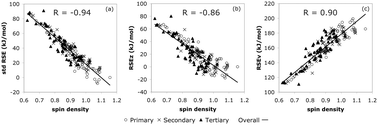

 Please wait while we load your content...
Please wait while we load your content...