Molecular dynamic simulations of OH-stretching overtone induced photodissociation of fluorosulfonic and chlorosulfonic acid†
Abstract
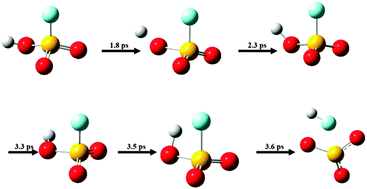
We investigate the OH-stretching overtone dynamics of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
O
- This article is part of the themed collection: Chemical Dynamics of Large Amplitude Motion

 Please wait while we load your content...
Please wait while we load your content...