Complementarity between high-energy photoelectron and L-edge spectroscopy for probing the electronic structure of 5d transition metal catalysts
Abstract
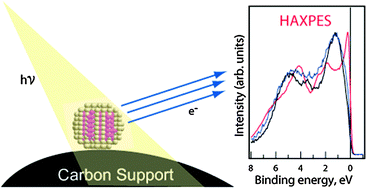
We demonstrate the successful use of hard
- This article is part of the themed collection: Recent developments in X-ray absorption spectroscopy

 Please wait while we load your content...
Please wait while we load your content...