CDASE—A reliable scheme to explain the reactivity sequence between Diels–Alder pairs†
Abstract
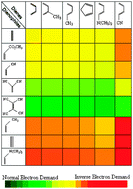
The reliability of the Comprehensive Decomposition Analysis of Stabilization Energy (CDASE) scheme, proposed recently (Phys. Chem. Chem. Phys., 2009, 11, 8306), has been demonstrated in the present study. Reactivity sequence among more than 100 pairs, taking part in Diels–Alder (DA) reaction, is successfully generated by this scheme. The diene series consisted mainly of cis-1,3-butadiene and different substituted butadienes whereas dienophiles are mainly ethylene and its different substitutions. Both the positive energy component (i.e., the energy parameter defined as ‘internal assistance’) and the negative energy component could generate the expected reactivity trend among the chosen DA pairs, which is also supported by the global electrophilicities of dienes and dienophiles and the corresponding charge-transfer values (ΔN). The numerical values of these components are capable of predicting even the ‘normal electron demand’ (NED) and ‘inverse electron demand’ (IED) nature of the corresponding DA reaction. The method is also capable of reproducing the lower reactivity of acetylene as dienophile when compared to that of ethylene. The reason for the success of CDASE-scheme in explaining intermolecular reactivity sequence is also analysed.

 Please wait while we load your content...
Please wait while we load your content...