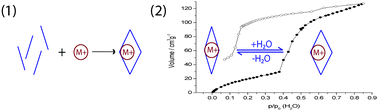
The influence of the guest ion on the synthesis of the flexible anionic open framework NaLa[(PO3H)2CH-C6H4-CH(PO3H)2]·4H2O, NaLa(H4L), was investigated. In a high throughput screening of the synthesis parameters, Na+ was identified as a strong structure-directing agent (SDA) for this framework. In addition, a series of compounds MLa(H4L) with M = Li+, Na+, K+, NH4+ and Rb+ were prepared by synthesis or ion exchange and characterized using X-ray powder diffraction, elemental analysis and scanning electron microscopy. Sorption of N2, Kr and H2O in these compounds revealed a preference of the network for water molecules. Furthermore, a strong influence of the guest ions on the shape of the water sorption isotherms could be observed. The isotherms of LiLa(H4L), NaLa(H4L) and KLa(H4L) show one distinctive step of adsorption that can be correlated with the expansion of the flexible framework upon hydration. The required partial pressure for the pore expansion depends on the incorporated guest ion and increases with the decreasing hydration enthalpy from Li+ to K+.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...