Rebecca R. Midtkandal, Simon J. F. Macdonald and Marie E. Migaud
Chem. Commun., 2010,46, 4538-4540
DOI:
10.1039/C0CC00467G,
Communication
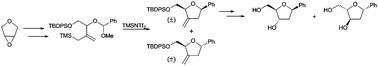
A novel synthetic procedure has been developed that provides access to D/L-2-deoxy-C-nucleosides from