An efficient asymmetric synthesis of the potent β-blocker ICI-118,551 allows the determination of enantiomer dependency on biological activity†‡
Abstract
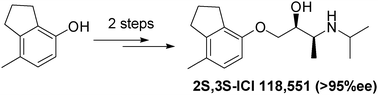
A highly efficient, practical and flexible two-step asymmetric synthesis of the β2-selective β-blocker ICI 118,551 is reported, allowing an unambiguous determination of the dependency of biological activity with optical activity, revealing the

 Please wait while we load your content...
Please wait while we load your content...