Transaminations with isopropyl amine: equilibrium displacement with yeast alcohol dehydrogenase coupled to in situcofactorregeneration†‡
Abstract
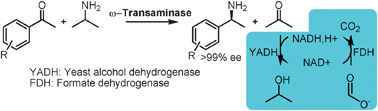
Enantiopure chiral amines synthesis using ω-transaminases is hindered by an unfavourable equilibrium, but when using
- This article is part of the themed collection: Enzymes and Proteins

 Please wait while we load your content...
Please wait while we load your content...