Interfacial tension between a complex coacervate phase and its coexisting aqueous phase
Abstract
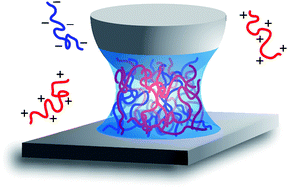
Complex coacervation is the associative phase separation in a solution of positively and negatively charged macroions. Despite the widespread use of coacervation in e.g. micellar

 Please wait while we load your content...
Please wait while we load your content...