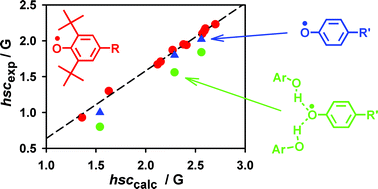
DFT calculations using the B3LYP functional, medium-sized basis sets and empirical scaling of the results provide quantitative estimates of the hydrogen isotropic hyperfine splitting constants (hscs) in 2,6-di-alkyl phenoxyl radicals (1–11). Literature hscs for phenoxyl (12), 4-methylphenoxyl (13) and 4-methoxyphenoxyl (14) radicals, on the other hand, are poorly predicted by using this method. This different behaviour is explained considering that experimental hscs of 12–14 are influenced by H-bonds formed between phenoxyls and their parent phenols, usually present in large amounts in solution as radical precursors. This was confirmed experimentally by measuring the EPR spectra of 12–14 in the presence of increasing amounts of their parent phenols, and by calculating the hscs in the case of the formation of 1 : 1 and 1 : 2 complexes between these radicals and phenol. Relevance of these results to the choice of reference hscs as benchmarks for theoretical calculations and to kinetic and thermochemical determinations on unhindered phenoxyl radicals is discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...