Functionalized alkoxy arene diazonium salts from paracetamol†
Abstract
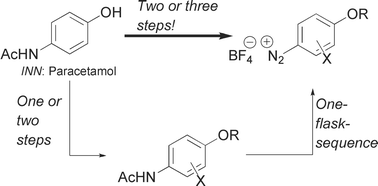
Arene diazonium tetrafluoroborates can be synthesized from aromatic acetamides via a sequence of deacetylation, diazotation and precipitation, induced by anion exchange. The reaction is conducted as a convenient one-flask transformation with consecutive addition of the appropriate reagents. Exchange of solvents or removal of byproducts prior to isolation of the product is not required. The arene diazonium salts are isolated from the reaction mixture by simple filtration. Two complementary protocols are presented, and the utility of the reaction is exemplified for a synthesis of the diarylheptanoid natural product de-O-methyl centrolobine.

 Please wait while we load your content...
Please wait while we load your content...