Unexpected transalkylation on 3-alkyl-2-alkylthio-1,3,4-thiadiazolium-5-thiolates: A computational and experimental mechanistic study†
Abstract
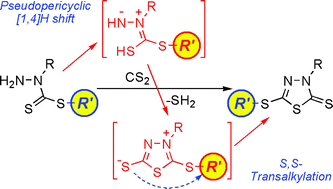
5-Alkylthio-3-methyl-2-thioxo-1,3,4-thiadiazolines have been obtained on heating alkyl 1-methyl-1-hydrazinecarbodithioates with CS2. A DFT-based computational mechanistic study suggests an initial pseudopericyclic [1,4]H shift as a key step, as well as the intermediacy of the otherwise expected isomers 2-alkylthio-3-methyl-1,3,4-thiadiazolium-5-thiolates, from which the final products are formed by stepwise S,S-transalkylation.

 Please wait while we load your content...
Please wait while we load your content...