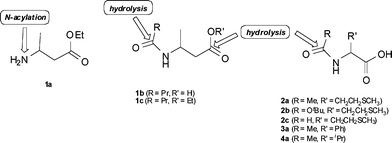
Various commercial lyophilized and immobilized preparations of lipase A from Candida antarctica (CAL-A) were studied for their ability to catalyze the hydrolysis of amide bonds in N-acylated α-amino acids, 3-butanamidobutanoic acid (β-amino acid) and its ethyl ester. The activity toward amide bonds is highly untypical of lipases, despite the close mechanistic analogy to amidases which normally catalyze the corresponding reactions. Most CAL-A preparations cleaved amide bonds of various substrates with high enantioselectivity, although high variations in substrate selectivity and catalytic rates were detected. The possible role of contaminant protein species on the hydrolytic activity toward these bonds was studied by fractionation and analysis of the commercial lyophilized preparation of CAL-A (Cat#ICR-112, Codexis). In addition to minor impurities, two equally abundant proteins were detected, migrating on SDS-PAGE a few kDa apart around the calculated size of CAL-A. Based on peptide fragment analysis and sequence comparison both bands shared substantial sequence coverage with CAL-A. However, peptides at the C-terminal end constituting a motile domain described as an active-site flap were not identified in the smaller fragment. Separated gel filtration fractions of the two forms of CAL-A both catalyzed the amide bond hydrolysis of ethyl 3-butanamidobutanoate as well as the N-acylation of methyl pipecolinate. Hydrolytic activity towards N-acetylmethionine was, however, solely confined to the fractions containing the truncated form of CAL-A. These fractions were also found to contain a trace enzyme impurity identified in sequence analysis as a serine carboxypeptidase. The possible role of catalytic impurities versus the function of CAL-A in amide bond hydrolysis is further discussed in the paper.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...