An effective procedure for the synthesis of acid-sensitive epoxides: Use of 1-methylimidazole as the additive on methyltrioxorhenium-catalyzed epoxidation of alkenes with hydrogen peroxide†
Abstract
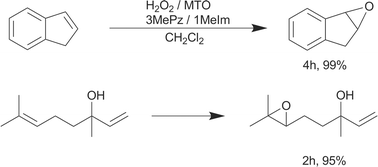
An effective method for suppression of ring opening and

 Please wait while we load your content...
Please wait while we load your content...