Reactions of a hydrido(hydrogermylene)tungsten complex with some heterocumulenes: hydrogermylation and thermal rearrangement†‡
Abstract
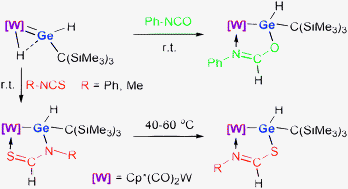
Reaction of a ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ge(H)[C(SiMe3)3] (1) with
Ge(H)[C(SiMe3)3] (1) with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond to give a W–Ge–O–C–N five-membered chelate complex. In contrast, the reactions of 1 with isothiocyanates RNCS (R = Ph, Me) led to hydrogermylation at the C
O bond to give a W–Ge–O–C–N five-membered chelate complex. In contrast, the reactions of 1 with isothiocyanates RNCS (R = Ph, Me) led to hydrogermylation at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond to yield W–Ge–N–C–S five-membered chelate complexes. In both cases, the most electronegative atom in the
N bond to yield W–Ge–N–C–S five-membered chelate complexes. In both cases, the most electronegative atom in the
- This article is part of the themed collection: Main group elements

 Please wait while we load your content...
Please wait while we load your content...