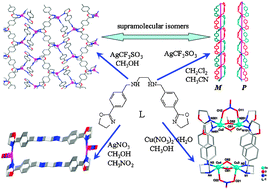
Six new coordination complexes, [AgL]CF3SO3 (1 and 2), [Ag2L2](NO3)2·4CH3NO2 (3), [AgL]NO3·0.5CH3OH (4), [AgL]BF4 (5) and [Cu4L2(OCH3)2(OH)2(NO3)2](NO3)2·0.32H2O (6), were obtained by reactions of the corresponding metal salts with ligand N,N′-bis[4-(4,5-dihydrooxazol-2-yl)-benzyl]ethane-1,2-diamine (L). The results of X-ray crystallographic analysis indicate that complexes 1 and 2 are supramolecular isomers with a wavelike cationic two-dimensional network structure of 1 and a double-stranded helical chain of 2. Complexes 4 and 5 also have helical chain structures with P and M forms. Differing from polymeric structure of 1, 2, 4 and 5, complexes 3 and 6 are a M2L2 molecular rectangle and a M4L2 cage, respectively. The results show that the flexible ligand L can have different conformation and coordination modes leading to complexes with varying structures. Magnetic data show that there are antiferromagnetic interactions in the dimeric unit of complex 6.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...