Substrate recognition and transport behavior analyses of amino acid antiporter with coarse-grained models†
Abstract
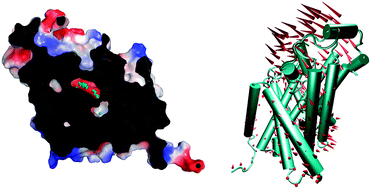
The L-arginine (Arg)/agmatine (Agm) antiporter AdiC is a vital transport protein of the arginine-dependent extreme acid resistance system of enteric bacteria. Recently, both substrate-free and Arg-bound structures of AdiC were determined by X-ray crystallography. In this article, the two different proteins were investigated with three simple models. Gaussian network model provided the information of conformational changes. It is found that Arg binding induces structural rearrangement in the extracellular domain, and transmembrane helix 6 (TM6) has the most pronounced trend of conformational changes. The moving directions of fluctuation regions were further ascertained by using anisotropy elastic network model and cross-correlation analysis. Interestingly, the two substrate-binding sites hypothesis of AdiC was confirmed directly by molecular docking. Furthermore, the binding preferences of these two sites were explained from the aspects of electrostatic complementarity and geometric matching. These simple coarse-grained analyses can be used as a general and quick method for the mechanism studies of transport proteins.

 Please wait while we load your content...
Please wait while we load your content...