Quantitative proteome and phosphoproteome analyses of cultured cells based on SILAClabeling without requirement of serum dialysis†
Abstract
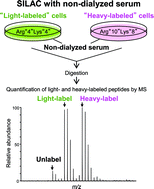
The use of dialyzed serum is essential in the application of the conventional stable
* Corresponding authors
a
Institute for Advanced Biosciences, Keio University, Daihoji, Tsuruoka, Yamagata 997-0017, Japan
E-mail:
y-ishi@ttck.keio.ac.jp
Fax: +81 235 29 0536
Tel: +81 235 29 0571
b PRESTO, Japan Science and Technology Agency, Sanbancho Bldg., 5-Sanbancho, Chiyodaku, Tokyo 102-0075, Japan
The use of dialyzed serum is essential in the application of the conventional stable

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
K. Imami, N. Sugiyama, M. Tomita and Y. Ishihama, Mol. BioSyst., 2010, 6, 594 DOI: 10.1039/B921379A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
