Molecular design and QSAR study of low acute toxicity biocides with 4,4′-dimorpholyl-methane core obtained by microwave-assisted synthesis†
Abstract
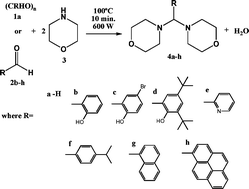
The solventless microwave-assisted synthesis of aldehydes (1a, 2b–h) and
* Corresponding authors
a Posgrado, Instituto Mexicano del Petróleo, Eje Central Lázaro Cárdenas No. 152, Apartado Postal 14-805, México, D.F.
b Programa de Ingeniería Molecular, Instituto Mexicano del Petróleo, Eje Central Lázaro Cárdenas No. 152, Apartado Postal 14-805, México, D.F.
c
Laboratorio de Síntesis Química y Electroquímica, Instituto Mexicano del Petróleo, Eje Central Lázaro Cárdenas No. 152, Apartado Postal 14-805, México, D.F.
E-mail:
lzamudio@imp.mx
d Departamento de Biotecnología, DCBS, Universidad Autónoma Metropolitana-Iztapalapa, Apartado Postal 55-535, México, D.F.
e Centro de Investigaciones Químicas, Universidad Autónoma del Estado de Morelos, Av. Universidad 1001, Cuernavaca, México
f
Departamento de Ciencias Naturales, DCNI, Universidad Autónoma Metropolitana-Cuajimalpa, Pedro Antonio de los Santos 84, Sn. Miguel Chapultepec, México, D.F.
E-mail:
hbeltran@correo.cua.uam.mx
The solventless microwave-assisted synthesis of aldehydes (1a, 2b–h) and

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
R. Hernández-Altamirano, V. Y. Mena-Cervantes, S. Perez-Miranda, F. J. Fernández, C. A. Flores-Sandoval, V. Barba, H. I. Beltrán and L. S. Zamudio-Rivera, Green Chem., 2010, 12, 1036 DOI: 10.1039/B905153H
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
